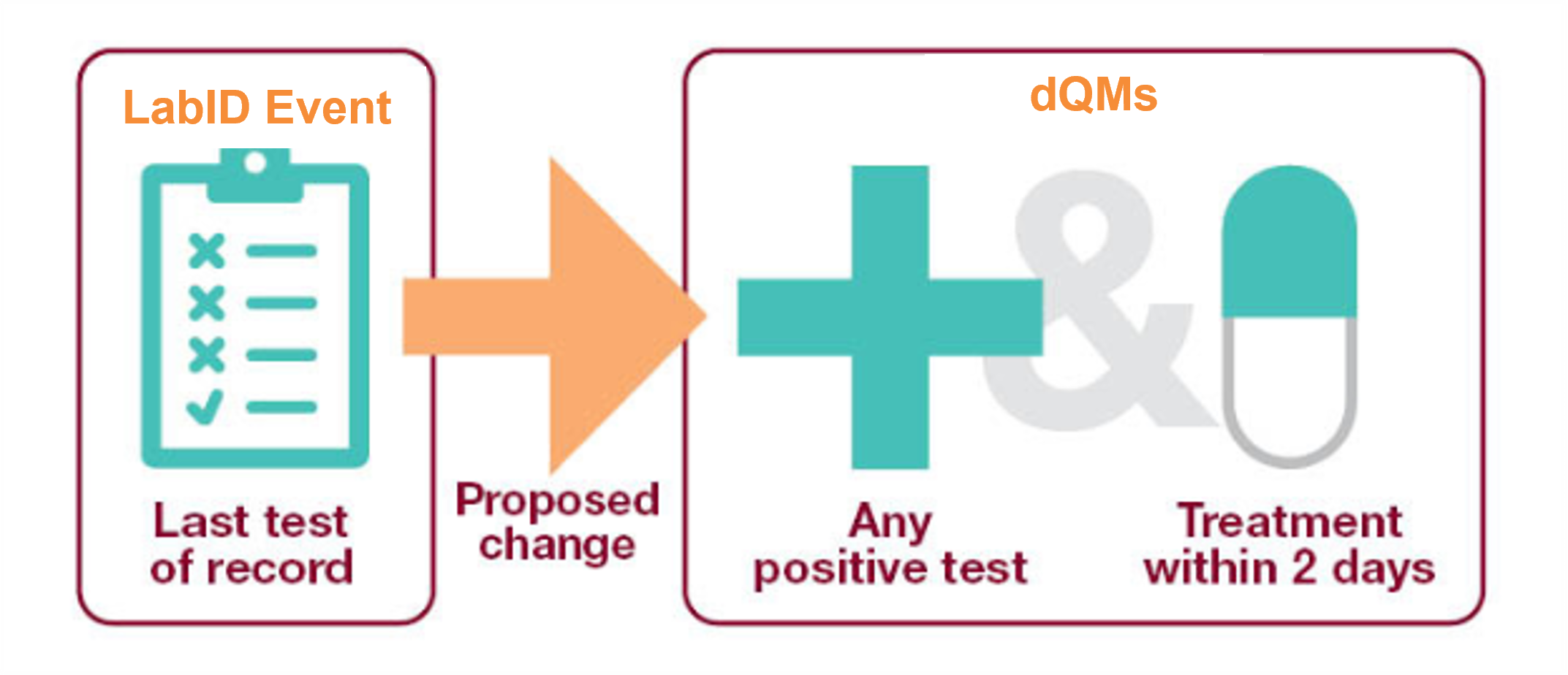
There are many different ways of testing for C. difficile infections. Laboratories which follow a multistep algorithm recognize that a positive toxin result is necessary to define clinical disease. Algorithm testing with GDH antigen or NAAT, followed by an EIA toxin test to confirm the active disease, is considered the best-performing algorithm. An efficient approach is to screen samples simultaneously for GDH and toxins A & B using an assay that includes both targets in one test— the C. DIFF QUIK CHEK COMPLETE® test
Properly distinguishing between true infections and carriers is crucial to protecting patients from unnecessary treatments. Rapid and accurate identification of toxigenic C. difficile allows for appropriate treatment when needed, allowing patients to recover their health, reconnect with loved ones, and reclaim their quality of life.