Legionella pneumophila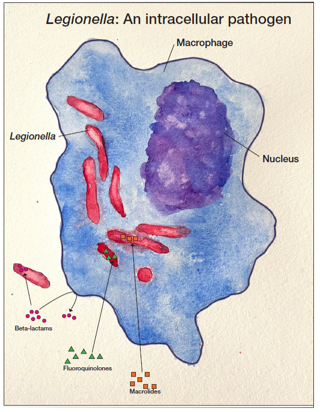
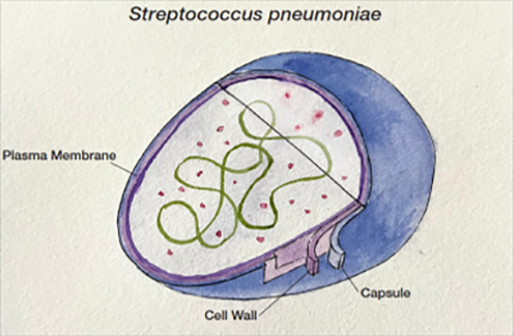
Legionella bacteria are aerobic, unencapsulated, Gram-negative, rod-shaped, intracellular pathogens (Figure 2). Although occurring naturally in small amounts in freshwater environments such as streams and lakes, Legionella poses a health risk when found in water distribution systems of public buildings such as hospitals and hotels. Legionella is also resistant to standard water disinfection procedures and can be found in treated potable water, hot tubs and nearly any water storage device.
These environments allow for Legionella proliferation and the organisms are then transmitted to humans via aerosolization or aspiration of the contaminated water. There are more than 50 Legionella species described in the literature, and 18 of the species have been associated with human infections. Legionella pneumophila is the cause of more than 90% of all cases of Legionnaires’ disease. L. pneumophila is divided into 15 serogroups, with serogroup 1 causing more than 84% of global reported cases of L. pneumophila legionellosis, though this figure is complicated by the fact that some tests only detect serogroup 1 and serogrouping is not routinely performed in many localities.20
Testing
Culture
The gold standard for diagnosis remains traditional culture-based methods, using respiratory samples or blood cultures. The most clear-cut advantage of culture is that positive results provide strong confirmation of organism, which can then be serogrouped or tested for antibiotic resistance. However, obtaining high quality sputum samples is a common limitation,21 such that negative results are typically not informative, particularly in Legionellosis, where less than 50% of patients produce sputum.22-25 Additionally, samples should be collected from the lower airway as upper airway samples can be contaminated.
Blood cultures from patients with pneumonia identify a bacterial pathogen in only 5-16% of cases. This finding is reasonable as not all patients with pneumonia are bacteremic. S. pneumoniae is detected in one third of positive blood cultures.26 Culture results will also be negative for viral pathogens, which appear to cause 30-50% of cases.26 The recent Guidelines of the ATS/IDSA do not recommend blood cultures for adults with nonsevere CAP who are managed as outpatients.21 Prompt initiation of appropriate antibiotic therapy is a goal of treatment for pneumonia illnesses. Culture requires more than 24 hours to identify an organism, or as long as 3-5 days for Legionella, and a further 1-2 days to provide information on its antibiotic sensitivity.27 If antibiotics have been started prior to specimen collection, culture becomes further compromised.